Research
We are an interdisciplinary lab, working at the interface of Biochemistry, Cell Biology, and Physics. Our work is driven by a sense of curiosity, fascination, and discovery. We study how biological systems, from molecules to organisms, achieve remarkable complexity through self-assembly, where local interactions give rise to emergent order. By combining quantitative experiments, theory, and computational modeling, we aim to uncover universal principles governing self-organization across scales.

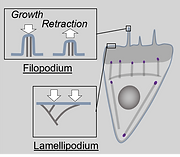
Molecular Self-assembly
Multicomponent mechanochemical regulation of actin dynamics
Cellular actin dynamics are essential in key processes such as cell migration, wound healing, division, and endocytosis. Living cells rapidly assemble and remodel their actin cytoskeleton in response to external signals to move, change shape, and reorganize their internal architecture. Cells can locally tune actin dynamics to meet the demands of specific biological processes, but how is this achieved in the crowded, heterogeneous cytoplasm? The central hypothesis driving our research is that intracellular actin dynamics emerges from an interplay between multicomponent biochemical factors (e.g., proteins) and mechanical forces. By reconstituting minimal actin networks and probing their dynamics in vitro, we seek to decode the rules of self-organization that enable actin dynamics in vivo
Our biophysical and biochemical toolbox


Total internal reflection microscopy (2 Nikon E-TIRFs + 1 iLas ring TIRF scopes)
Microfluidics (Automated flow control systems)
Optical tweezers (Lumicks C-Trap confocal setup)
Magnetic Tweezers
Multispectral single-molecule imaging

